Challenge Models
Challenge models are developed to mimic real-life infection and disease development. Our models are well-established and reproducible. Our wet lab is equipped with state-of-the-art non-recirculation trial units and the tank design can be tailored with regards to individual light and feeding regimes, temperature control and salinity adjustments.
Contact us
Virus
Infectious salmon anemia virus (ISAV) - Infectious salmon anemia (ISA)
Introduction
Infectious salmon anemia (ISA) is a systemic disease of farmed Atlantic salmon. Disease outbreaks have been reported in Norway, Canada, Scotland, the Faroe Islands, USA and Chile. Prophylactic measures are sought to prevent outbreaks of ISA in the salmon aquaculture industry. VESO Aqualab offers challenge models to evaluate the efficacy of vaccines, the effect of prophylactic feeding and the effect of selective breeding for resistance.
Challenge models to evaluate the effect of vaccination
The fish will be vaccinated at the parr stage and either photoperiod-manipulated to smoltify or maintained as pre-smolts. After vaccination the fish are either challenged by i.p. injection of virus or by cohabitation with i.p. injected shedder fish. Evaluation of the protection provided by the vaccines is based on differences in mortality in vaccinated and unvaccinated fish after challenge.
Challenge models to evaluate genetic resistance profiles
Fish of different genetic characteristics can be kept in separate tanks or mixed in one tank during challenge. Possible tank effects can be reduced by mixing all families in one tank. The fish will be acclimatized for minimum two weeks followed by challenge by i.p. injection of virus or cohabitation with i.p. injected shedder fish. Subpopulations of fish from the challenged fish pool are typically identified by DNA fingerprinting or PIT-tag readings.
Challenge models to evaluate the effect of feeding
The fish will be acclimatized for minimum two weeks followed by a period of test feeding. After challenge by i.p. injection or cohabitation, mortality will be recorded throughout a five weeks observation period.

Piscine myocarditis virus (PMCV) - Cardiomyopathy syndrome (CMS)
Challenge model
Piscine myocarditis virus (PMCV) is the causative agent of cardiomyopathy syndrome (CMS). VESO Aqualab has established an experimental challenge model for infection with PMCV. Smolts can be challenged through the intraperitoneal or intramuscular route. The diagnosis is typically based on qPCR and histopathology, and trials are run for 8 – 9 weeks post challenge. There is a strong correlation between viral load and histopathological changes in the heart.
Inoculum
Cultivation of PMCV in cell line systems has so far not been successful. Experimental transmission of CMS has therefore been demonstrated using tissue homogenate from CMS-diagnosed fish. The challenge material produced at VESO Aqualab origins from a highly virulent field outbreak of CMS. The homogenate was screened for all known pathogens by qPCR. It was thereafter passed repeatedly through our pathogen free test fish to ensure a tissue homogenate containing PMCV only.



Piscine orthoreovirus (PRV) - Heart and skeletal muscle inflammation (HSMI)
Introduction
Piscine orthoreovirus (PRV) is the causative agent of heart and skeletal muscle inflammation (HSMI). The virus is ubiquitous, but there is a significant increase in the viral load during an outbreak of HSMI. Experimental transmission of HSMI has been demonstrated using tissue homogenate, red blood cells and purified virus. The diagnosis is based on slowly emerging histopathological changes in the heart. qPCR and immunohistochemistry can be used to detect the virus at early stages of infection, before the onset of histopathological changes.
Challenge model
A challenge model for experimental infection of Atlantic salmon has been developed at VESO Aqualab in close collaboration with researchers from the Norwegian School of Veterinary Science (NMBU). Parr and smolts can be challenged by i.p. injection of infectious material or by cohabitation, followed by serum and tissue sampling. Cohabitation trials are typically run for 8-10 weeks. There is a strong correlation between viral load and the following histopathological changes in the heart.

Salmon pancreatic disease virus (SPDV - SAV2/3) - Pancreas disease (PD)
Introduction
Salmon pancreas disease virus (SPDV, belonging to the genus Alphaviridae) is the causative agent of pancreas disease (PD) in farmed Atlantic salmon. VESO Aqualab offers challenge models with SAV2 and SAV3 to evaluate the efficacy of vaccines, the effect of selective breeding for resistance and the effect of prophylactic feeding and recovery. The outcome parameters are typically viral load (measured by qPCR) and histopathological changes in the heart during the acute phase of the disease, and pancreas pathology and growth/condition factor during the chronic phase of the disease. Mortality in parr/smolts is typically low and variable and is not recommended as the principal outcome parameter. Mortality in fry is normally 30-60% and usually consistent.
Challenge models to evaluate effect of vaccination
The fish will be vaccinated at the parr stage, immunized and experimentally infected with SPDV by i.p. injection or cohabitation in freshwater or seawater.
Challenge models to evaluate the effect of feeding
The fish will be acclimatized for minimum two weeks followed by a period of test feeding. After challenge by i.p. injection or cohabitation, sampling for histopathological analysis is done throughout the observation period. Fish growth is essentially absent in unprotected trial groups from 2 to 10 weeks post-challenge.
Challenge models to evaluate the effect of selective breeding
Salmon fry/parr/smolt with different genetic characteristics should preferably be mixed in one tank to reduce possible tank effects. The fish are usually acclimatized for minimum two weeks followed by water borne challenge. Mortality is monitored through a six- to ten week period. Subpopulations of fish from the challenged fish pool are typically identified by DNA fingerprinting.

Infectious pancreatic necrosis virus (IPNV) - Infectious pancreatic necrosis (IPN)
Introduction
The susceptibility to IPNV infection is highly dependent on the genetic characteristics of the test fish. Atlantic salmon are susceptible to IPN by experimental infection as first feeding fry (fresh water) and as post-smolts (sea water). A Norwegian IPNV strain originating from an outbreak in post-smolts is used for all challenges. The virus is a serotype Sp/N1, characterized as highly virulent and carrying putative molecular virulence marker motifs.
Cohabitation challenge of smolts (sea water)
Pre-smolts to be included in a bath or cohabitant challenge study are photoperiod-manipulated to smoltify. Challenges can be performed within 1-3 weeks after transfer to sea water. For cohabitant challenge studies, 20% i.p. injected shedders are introduced to the challenge tank. Fish are observed throughout a <35-day period.
Immersion challenge of fry (fresh water)
Fish of different genetic characteristics can be kept in separate tanks or mixed in one tank during challenge. Possible tank effects can be reduced by mixing all families in one tank. The fish (0.1-0.2 grams in average weight) will be challenged by immersion 1-3 weeks after onset of start feeding. Mortality is recorded for 35-50 days. Subpopulations of fish from the challenged fish pool are typically identified by DNA fingerprinting.

Bacterium
Yersinia ruckeri - Yersiniosis / Enteric redmouth disease (ERM)
Introduction
Yersiniosis or enteric red mouth disease (ERM) is caused by the gram-negative bacterium Yersinia ruckeri. The disease was historically observed in smaller fish in fresh water, but during the last years several outbreaks in larger fish in sea water have been reported. Fish may suffer from sub clinical infections and outbreaks can be seen after stressful situations such as transport or delousing.
Challenge models
VESO Aqualab offers challenge models for fry, parr and smolts with Y. ruckeri serotype O1 isolates representative for fresh water and sea water outbreaks. Fish are challenged by i.p. injection or immersion.

Vibrio ordalii - Vibriosis
Introduction
Bacteria belonging to the genus Vibrio are the causative agents of vibriosis. Vibriosis can cause significant mortality once an outbreak is in progress and vaccination is used to minimize the impact of the disease. VESO Aqualab offers challenge models in salmon with:
- Vibrio salmonicida (causative agent of cold-water vibriosis or Hitra-disease)
- Vibrio anguillarum, serotype O1 and O2a (causative agents of vibriosis)
- Vibrio ordalii (causative agent of vibriosis)
Challenge models to evaluate the effect of vaccination
Salmon parr (pre-smolts) are acclimatized for a minimum of one week before vaccination. After the immunization period fish are challenged by i.p. injection or immersion in fresh water or sea water. After challenge, mortality is recorded during an observation period of two to four weeks. Evaluation of the potency of the vaccine is based on differences in mortality in vaccinated and unvaccinated fish.
Vibrio ordalii:

Vibrio Anguillarum:

Aeromonas salmonicida - Furunculosis
Introduction
Aeromonas salmonicida is the causative agent of furunculosis. This gram-negative bacterium infects salmonid species worldwide, with both typical and atypical strains causing disease. All salmonids are believed to be naturally susceptible to infection with Aeromonas salmonicida, and the bacterium has been isolated from a range of other species as well. VESO offers challenge models with Aeromonas salmonicida in Atlantic salmon and rainbow trout.
Challenge models to evaluate the effect of vaccination
Salmonid parr (pre-smolts) are acclimatized for a minimum of one week before vaccination. After the immunization period fish are challenged by i.p. injection or cohabitation with i.p. injected shedder fish. After challenge, mortality is recorded during an observation period of three to four weeks. Evaluation of the potency of the vaccine is based on differences in mortality in vaccinated and unvaccinated fish.

Atypical Aeromonas salmonicida - Atypical furunculosis
Information coming soon.
Francisella sp. - Francisellosis
Introduction
Francisella is an intracellular bacterium that causes the systemic granulomatous inflammatory disease francisellosis in cod. VESO Aqualab offers challenge models with Francisella sp.
Challenge models to evaluate the effect of vaccination
Cod are acclimatized for a minimum of one week before challenge by i.p. injection or immersion. After challenge, mortality is recorded during an observation period of two to three weeks. Evaluation of the potency of the vaccine is based on differences in mortality in vaccinated and unvaccinated fish.
Diagram 1: Challenge by intraperitoneal injection

Diagram 2: Challenge by immersion

Phocoenobacter sp. (previously Pasteurella sp.)
Introduction
Bacteria belonging to the large family of Gram-negative bacteria, Phocoenobacter (from the family Pasteurellaceae) are the causative agents of pasteurellosis (Nilsen et. al., 2025). The bacteria are capable of inducing clinical disease in several aquatic fish species, such as lumpfish and Atlantic salmon during the marine production phase. The problem has escalated in recent years and a range of geno- and serotypes with different disease profiles have been described. VESO Aqualab has established challenge models in Atlantic salmon with Phocoenobacter sp. isolated from recent field outbreaks in Norway and Scotland. The work has been done in close collaboration with the Norwegian Veterinary Institute.
Challenge model
Two Phocoenobacter sp. are available, Phocoenobacter skyensis (isolate 11741) and Phocoenobacter atlanticus (isolate 11639). Atlantic salmon post-smolts are acclimatized for minimum two weeks in sea water before challenge by i.p. injection of bacteria at 15°C. Mortality is recorded during an observation period of 2-3 weeks.
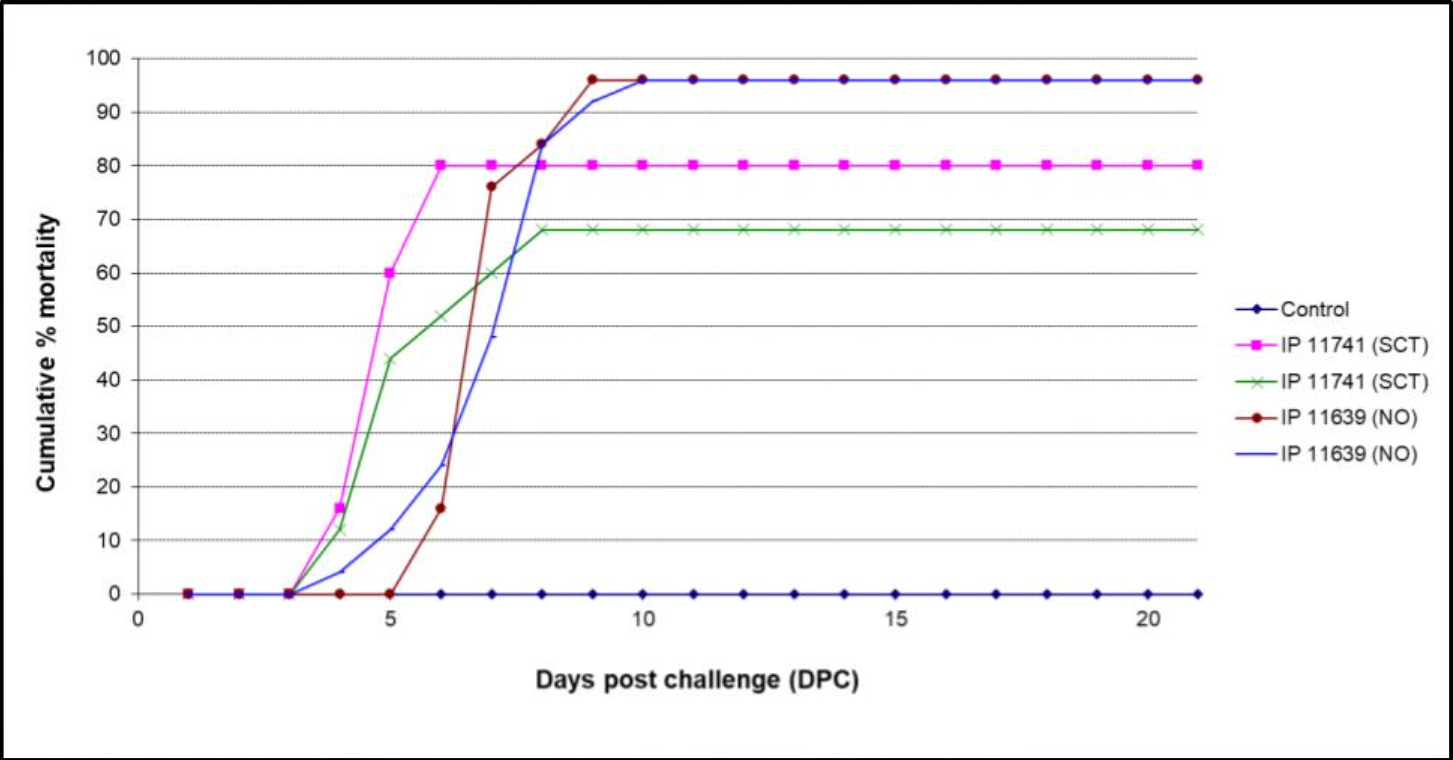
Available models

References
Hanne K. Nilsen, Anne Berit Olsen, Thomas H. Birkbeck, Farah Manji, Duncan J. Colquhoun and Snorre Gulla. Reclassification of Pasteurella skyensis as Phocoenobacter skyensis comb. nov. and description of Phocoenobacter atlanticus sp. nov. isolated from diseased Atlantic salmon (Salmo salar) and lumpfish (Cyclopterus lumpus), with subdivision into Phocoenobacter atlanticus subspecies atlanticus subsp. nov. and Phocoenobacter atlanticus subspecies cyclopteri subsp. nov..
International Journal of Systematic Evolutionary Microbiology 2025;75:006729 DOI 10.1099/ijsem.0.006729
Pasteurella sp. - Pasteurellosis
Introduction
Bacteria belonging to the family Pasteurellaceae are the causative agents of pasteurellosis. High mortality by pasteurellosis has been reported in Norway since 2012. Mortality related to the outbreak of Pasteurella sp. occurs often 2-4 weeks after episodes that involve a lot of stress for the fish, such as vaccination, transport and exposure in the cage. Mortality can accumulate to high levels if the disease is not treated. VESO Aqualab offers challenge models with Pasteurella sp.
Challenge model to evaluate the efficacy of vaccination
Lumpfish are acclimatized for a minimum of two weeks before vaccination. After an immunization period of four weeks fish are challenged by i.p. injection or cohabitation. After challenge, mortality is recorded during an observation period of 2-4 weeks. Evaluation of the potency of the vaccine is based on differences in mortality in vaccinated and unvaccinated groups

Photobacterium damselae - Pasteurellosis
Introduction
Pasteurellosis is caused by the gram-negative and halophilic bacterium Photobacterium damselae. The disease affects a wide range of marine species, and fish can harbor the bacterium as subclinical infection for long periods of time. VESO Aqualab offers challenge models with Photobacterium damselae.
Challenge model to evaluate the efficacy of vaccination
Seabass (average weight 4-10 grams) are acclimatized to 20°C for a minimum of two weeks before vaccination. After a four-week immunization period, fish are challenged by i.p. injection. After challenge, mortality is recorded during an observation period of 21 days. Evaluation of the potency of the vaccine is based on differences in mortality in vaccinated and unvaccinated groups.

Piscirickettsia salmonis - Salmon rickettsial syndrome (SRS)
Introduction
Piscirickettsia salmonis is the etiological agent of Salmon rickettsial septicemia (SRS) or Piscirickettsiosis. The disease causes high mortality and significant economic losses to the Chilean salmon industry. Experimental trials are indispensable to evaluate the efficacy of prophylactic measures against Piscirickettsiosis, such as vaccination, selective breeding for resistance or functional feeds. VESO Aqualab offers experimental challenge models for pre-smolts (parr) and post-smolts. After challenge, the fish are observed daily, and mortality recorded. The diagnosis of Piscirickettsiosis is based upon presence of the characteristic external and internal signs of the disease and cultivation of the bacterium on agar plates. Serum and tissue can be sampled for subsequent analysis by ELISA and RT-qPCR.
Challenge of parr
Parr will be acclimatized to 15°C before challenge. Fish are challenged by i.p. injection of bacteria, or cohabitation with i.p. injected shedders. After challenge the fish are kept under close observation and mortalities registered.
Challenge of post-smolts
Pre-smolts to be included in an i.p. or cohabitant challenge study are photoperiod manipulated to smoltify before adaption to sea water. Fish will be acclimatised to 15°C before challenge. If the fish are included in a vaccination trial, vaccination will be performed during the smoltification period. The test fish are challenged after transfer to sea water, either by i.p. injection of bacteria or by cohabitation with i.p. injected shedder fish.

Vibrio anguillarum O1 - Vibriosis
Introduction
Bacteria belonging to the genus Vibrio are the causative agents of vibriosis, which can be a problem in cod farming. Vibriosis can cause significant mortality once an outbreak is in progress and vaccination is used to minimize the impact of the disease. VESO Aqualab offers challenge models with Vibrio anguillarum, serotypes O1, O2a and O2b.
Challenge models to evaluate the effect of vaccination
Cod are acclimatized for a minimum of one week before challenge by i.p. injection or immersion. After challenge, mortality is recorded during an observation period of two to three weeks. Evaluation of the potency of the vaccine is based on differences in mortality in vaccinated and unvaccinated fish.


Vibrio anguillarum O2a - Vibriosis
Introduction
Bacteria belonging to the genus Vibrio are the causative agents of vibriosis. Vibriosis can cause significant mortality once an outbreak is in progress and vaccination is used to minimize the impact of the disease. VESO Aqualab offers challenge models in salmon with:
- Vibrio salmonicida (causative agent of cold-water vibriosis or Hitra-disease)
- Vibrio anguillarum, serotype O1 and O2a (causative agents of vibriosis)
- Vibrio ordalii (causative agent of vibriosis)
Challenge models to evaluate the effect of vaccination
Salmon parr (pre-smolts) are acclimatized for a minimum of one week before vaccination. After the immunization period fish are challenged by i.p. injection or immersion in fresh water or sea water. After challenge, mortality is recorded during an observation period of two to four weeks. Evaluation of the potency of the vaccine is based on differences in mortality in vaccinated and unvaccinated fish.
Diagram 1:

Diagram 2:

Vibrio anguillarum - Vibriosis
Introduction
Bacteria belonging to the genus Vibrio are the causative agents of vibriosis. Vibriosis can cause significant mortality once an outbreak is in progress and vaccination is used to minimize the impact of the disease. VESO Aqualab offers challenge models with Vibrio anguillarum.
Challenge model to evaluate the efficacy of vaccination
Lumpfish are acclimatized for a minimum of two week before vaccination. After an immunization period of five to six weeks, fish are challenged by i.p. injection. After challenge, mortality is recorded during an observation period of maximum 21 days. Evaluation of the potency of the vaccine is based on differences in mortality in vaccinated and unvaccinated groups.

Mortality in groups of unvaccinated lumpfish i.p. injected with increasing doses of Vibrio anguillarum.
Vibrio salmoncida - Cold water vibriosis / Hitra disease
Introduction
Vibrio salmonicida is the causative agent of cold water vibriosis or so-called Hitra disease. During 2012 VESO Aqualab received several requests regarding outbreaks of this disease in northern Norway. We brushed the dust off our old challenge model, and further developed it into a clinically relevant bath challenge model. Mortality in control smolts typically reaches 70% and a new field isolate from northern Norway is available for challenge.
Challenge models to evaluate the effect of vaccination
Salmon parr (pre-smolts) are acclimatized for a minimum of one week before vaccination. After the immunization and smoltification period fish are challenged by i.p. injection or immersion in fresh water or sea water. After challenge, mortality is recorded during an observation period of two to four weeks. Evaluation of the potency of the vaccine is based on differences in mortality in vaccinated and unvaccinated fish.

Moritella viscosa - Winter ulcers
Introduction
Moritella viscosa is a gram-negative bacterium that is the causative agent of winter ulcer disease. Winter ulcers often appear during periods of cold water and high salinity and the disease is characterized by the formation of dermal and subdermal ulcers. When the temperature increases, or salinity decreases, fish may recover from an outbreak of winter ulcers.
Challenge by immersion
VESO Aqualab has long experience in conducting challenge trials with Moritella viscosa, and we recommend models based on bath challenge of smolts in sea water which mimic a natural disease situation. Pre-smolts are photoperiod-manipulated to smoltify, transferred to sea water and acclimatized at 8-10°C before challenge. Development of ulcers and mortality is observed throughout a 15-30-day period. The mortality rate may be controlled by adjusting the water temperature.
Confirmatory diagnosis of Moritella viscosa is based on colony morphology and viscosity following growth on blood agar plates from smears from the pronephros of dead fish. Principal outcome parameters are prevalence and severity of ulceration and mortality.
Renibacterium salmoninarum - Bacterial kidney disease (BKD)
Introduction
Renibacterium salmoninarum is the causative agent of Bacterial Kidney Disease - BKD. This disease is broadly distributed within salmon farming countries, including Europe, North and South America.
BKD may occur as a chronic or acute clinical manifestation, producing important mortality levels causing economic losses in farming companies. In this scenario, it becomes critical to be able to test a variety of prophylactic measures such as vaccines, antimicrobials and genetic selection programs under controlled trial conditions.
VESO is offering an experimental challenge model fish at the stage of pre-smolts (parr). After challenge, the fish are observed daily and mortality is recorded. The diagnosis of the disease is based upon presence of the characteristic internal signs of the disease and cultivation of the bacterium in agar plates. The isolate was from a disease outbreak in Chile and the identity was confirmed by the Veterinærinstituttet, Norway.
Challenge
In preparation to the challenge, fish are acclimated to 15°C for a minimum of 7 days. Then, fish are challenged via i.p. injection with the challenge material. After challenge, the fish are kept under a minimum disturbance policy and under close observation for recording mortalities.
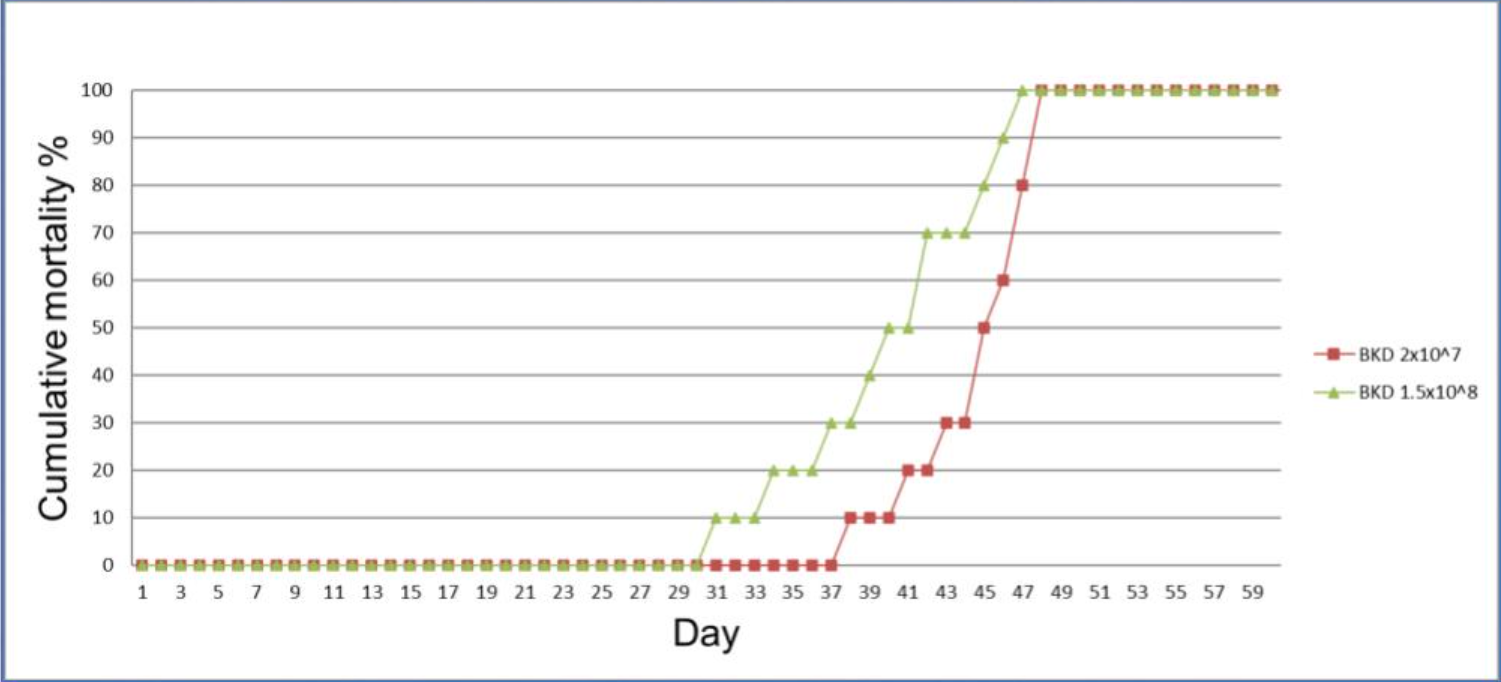
Available models
Parasite
Paramoeba perurans - Aemobic gill disease (AGD)
Introduction
Amoebic Gill Disease (AGD) has been a problem in Tasmanian aquaculture for years. The disease is caused by the amoeba Paramoeba perurans, which thrives very well in high salinity sea water at increasing temperatures. During the last years outbreaks have been reported in Ireland and Scotland, and also along the Norwegian coastline.
Challenge model
To face this problem, VESO Aqualab established a challenge model for AGD. Through close collaboration with the Norwegian Veterinary Institute, we are able to cultivate the amoeba and have successfully infected salmon smolts in experimental trials. We have studied the dynamics of the challenge model at different temperatures and salinities, as well as during fresh water treatment. Large scale AGD trials with a maximum of ~4000 smolts in one tank can be conducted.


Paramoeba perurans (Terje Steinum, NVI).
Atlantic salmon gills

Paramoeba perurans (Terje Steinum, NVI) and Atlantic salmon gills three weeks after bath challenge with Paramoeba perurans.
Sea lice (Lepeophtheirus salmonis)
Introduction
VESO has broad experience in design and conduct of experimental sea lice trials. Sea lice larvae and motile development stages are cultivated at VESO Vikan. They are typically produced from egg strings that have been obtained from fish farms. Larvae are hatched in specially designed units.
Challenge models
Sea lice challenge trials can be conducted to evaluate the efficacy of new generation anti parasitical drugs, vaccination, feeding or selective breeding for resistance against sea lice attachment. Challenges are typically performed using copepods.
Effect of in vivo treatment
VESO has established several challenge models for testing and screening of chemotherapeutics. Trials are performed with sea lice of all developmental stages (copepodite – adult) attached to seawater adapted post-smolts. We can also evaluate the hatching ability of egg strings after exposure to test substances.
Bioassay – in vitro
Bioassays are designed to measure the sensitivity of a sea lice population to a specific chemotherapeutic. Bioassays may be performed on planktonic larval stages or motile (pre-adult and adult) developmental stages. The sensitivity to test compounds will show a degree of variation depending on e.g. developmental stage and gender. Bioassays must therefore be individually designed according to each test substance.

Tendency of developmental drift in a laboratory sea lice population.
Do you have a question?
Contact us - we are happy to help.



